Background: The COVID pandemic has resulted in significant changes many aspects of daily living. To understand the impact that COVID-19 has had on the myeloproliferative neoplasm (MPN) patient population, we conducted an internet based survey.
Methods:
Survey: This survey was hosted Mayo Clinic's secured REDCap system for online surveys with a link to the survey alongside a brief description posted via the www.mpnqol.com website, as well as other MPN organizational partners. Survey responses were completely anonymous. Questions included MPN-Total symptom score (TSS), NCCN distress thermometer (NCCN-DT), questions regarding impact on medical care, and questions from CDC COVID-19 community survey question bank regarding the impact of social distancing as well as changes in health behaviors.
Distress thermometer, MPN-TSS, and/or questions related to the impact of COVID-19 on MPN treatment were analyzed by: MPN diagnosis, among those with ET, PV, or MF diagnoses; medication status; stay at home order; community spread; and country. Associations were tested using Kruskal-Wallis or Wilcoxon rank sum tests (for continuous variables) or Fisher Exact tests (for categorical variables).
Analysis:
Results:
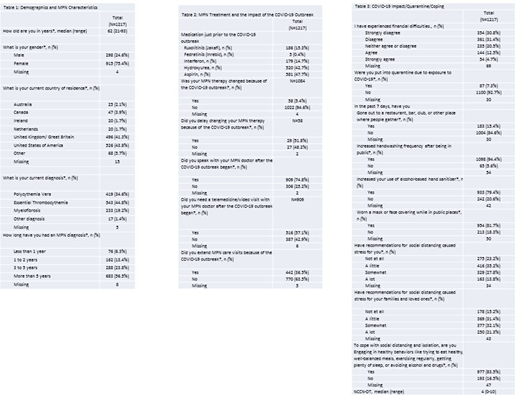
Patient Demographics (Table 1): 1560 people responded to the survey, 1217 were eligible for analysis. Median age was 62 (range: 21-93), and 298 (24.6%) respondents were male. There were respondents from USA, Australia, Canada, Netherlands, Ireland, and UK. 233 patients (19.2%) have myelofibrosis, 419 (34.6%) polycythemia vera and 543 (44.8%) essential thrombocythemia. At the time of the COVID outbreak, 1026 (84.3%) were on MPN directed medical therapy, including ruxolitinib (15.3%), interferon (14.7%), hydroxyurea (42.7%) and ASA (47.7%). 165 (13.6%) respondents received COVID testing, of which 5 had positive tests.
Impact on MPN Care (Table 2): We sought to understand how patient's clinical care changed. Over half respondents who spoke to their MPN doctor had a telemedicine visit after COVID19 (57.1%). 422 (36.5%) patients spaced out visits, of which 99 (22.7%) felt there were consequences. A change in therapy due to COVID-1 occurred in only 5.4% of patients.
MPN Symptom Burden and QoL: Data captured on the NCCN-DT had a median of 4 (0-10). MPN-SAF-TSS composite score was collected in 1150 respondents, median score was 26 (0-90). These scores are higher than those previously reported.
Pandemic Impact on Lifestyle (Table 1): 595 (49.5%) of patients report living in a community where there is significant COVID-19 spread. 946 (78.9%) reported that COVID-19 has impacted their day to day life. 198 (17.2%) of patients agreed, or strongly agreed that COVID had a significant impact on their finances. 799 (67.8%) had a stay at home order. Of those who quarantined (112), the median duration was 30d (1-120). The majority of people increased hand-washing, and cleaning habits. 954 (81.7%) respondents reported wearing masks in public. 908 (76.8%) reported increased stress from social distancing. The majority of respondents report using healthy coping habits, such as reaching out to friends/loved ones, breathing and relaxation, as well as healthy diet. Less than 15% of patients report unhealthy coping strategies, such as use of opioids, benzodiazepines, alcohol, or cannabis
Factors associated with response: There were no differences in responses based on type of MPN. If a patient was on medication, they were more likely to have spoken to their provider (p<0.001). If there was a stay at home order in place, there was a higher MPN-TSS score (p<0.001). If the respondent lived in an area of high community spread, they had a higher NCCN-DT score (p<0.001). Patients in the USA had a higher NCCN-DT score (p=0.001), were more likely to stretch out the duration of time between visits (p<0.001) and less likely to have a telemedicine visit (p<0.001). Although fewer respondents in the USA thought COVID-19 was a serious disease (p=0.02), a higher percentage of respondents wore masks (p <0.001).
Conclusions: In our survey of MPN patients, there were many changes noted in clinical care. The use of telemedicine was common, and at least a third had significant changes in their care. Most experienced increased stress, however, employed healthy coping strategies. Only a minority of patients had a COVID test, and only 5 were positive, further data will be needed to understand the impact of COVID infection.
Harrison:Roche: Honoraria; Incyte Corporation: Speakers Bureau; Celgene: Honoraria, Research Funding, Speakers Bureau; Sierra Oncology: Honoraria; Promedior: Honoraria; Shire: Honoraria, Speakers Bureau; AOP Orphan Pharmaceuticals: Honoraria; Novartis: Honoraria, Research Funding, Speakers Bureau; Janssen: Speakers Bureau; Gilead Sciences: Honoraria, Speakers Bureau; CTI Biopharma Corp: Honoraria, Speakers Bureau. Pemmaraju:Affymetrix: Other: Grant Support, Research Funding; Plexxikon: Research Funding; Incyte Corporation: Honoraria; Roche Diagnostics: Honoraria; Blueprint Medicines: Honoraria; Novartis: Honoraria, Research Funding; SagerStrong Foundation: Other: Grant Support; DAVA Oncology: Honoraria; Samus Therapeutics: Research Funding; Cellectis: Research Funding; Daiichi Sankyo: Research Funding; Celgene: Honoraria; AbbVie: Honoraria, Research Funding; Stemline Therapeutics: Honoraria, Research Funding; MustangBio: Honoraria; LFB Biotechnologies: Honoraria; Pacylex Pharmaceuticals: Consultancy. Vannucchi:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Blueprint: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Gupta:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol MyersSquibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Research Funding; Pfizer: Consultancy. Mascarenhas:Celgene, Prelude, Galecto, Promedior, Geron, Constellation, and Incyte: Consultancy; Incyte, Kartos, Roche, Promedior, Merck, Merus, Arog, CTI Biopharma, Janssen, and PharmaEssentia: Other: Research funding (institution). Shimoda:Kyowa Hakko Kirin Co., Ltd.: Research Funding; Pfizer Inc.: Research Funding; Otsuka Pharmaceutical: Research Funding; Celgene: Honoraria; Perseus Proteomics: Research Funding; PharmaEssentia Japan: Research Funding; AbbVie Inc.: Research Funding; Astellas Pharma: Research Funding; Merck & Co.: Research Funding; CHUGAI PHARMACEUTICAL CO., LTD.: Research Funding; Bristol-Myers Squibb: Honoraria; Takeda Pharmaceutical Company: Honoraria; Novartis: Honoraria, Research Funding; Shire plc: Honoraria; Asahi Kasei Medical: Research Funding; Japanese Society of Hematology: Research Funding; The Shinnihon Foundation of Advanced Medical Treatment Research: Research Funding. Kiladjian:AOP Orphan: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Komatsu:AbbVie: Other: member of safety assessment committee in M13-834 clinical trial.; Otsuka Pharmaceutical Co., Ltd., Shire Japan KK, Novartis Pharma KK, PharmaEssentia Japan KK, Fuso Pharmaceutical Industries, Ltd., Fujifilm Wako Pure Chemical Corporation, Chugai Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Takeda Pharmaceutica: Research Funding; Meiji Seika Pharma Co., Ltd.: Patents & Royalties: PCT/JP2020/008434, Research Funding; PPMX: Consultancy, Research Funding; Otsuka Pharmaceutical Co., Ltd., PharmaEssentia Japan KK, AbbVie GK, Celgene KK, Novartis Pharma KK, Shire Japan KK, Japan Tobacco Inc: Consultancy; Takeda Pharmaceutical Co., Ltd, Novartis Pharma KK, Shire Japan KK: Speakers Bureau. Abello:Takeda: Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Amgen: Consultancy, Research Funding; Dr. Reddy's: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding. Gomez-Almaguer:Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Scherber:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Mauro:Novartis: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding; Sun Pharma/SPARC: Research Funding; Takeda: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding; Pfizer: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding. Mesa:Samus Therapeutics: Research Funding; Novartis: Consultancy; Genentech: Research Funding; Incyte: Research Funding; Bristol Myers Squibb: Research Funding; AbbVie: Research Funding; CTI BioPharma: Research Funding; Sierra Oncology: Consultancy; LaJolla Pharmaceutical Company: Consultancy; Promedior: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal